Although most attention in the industry is currently centered on THC and CBD, minor cannabinoids (such as CBG, CBDV, THCV, and CBC) contribute to the overall mental and physical effects sought after by medical marijuana patients. In several studies, THC has been reported to bind to the CB1 and CB2 receptors1 found in the central and peripheral nervous system.2,3 Binding to these receptors generates either a psychoactive effect or a physical one such as an anti-inflammatory reaction.4 These effects can also be activated by the presence of other minor cannabinoids or even some terpenes such as β-caryophyllene.5,6
After repeated binding and signaling, CB1 receptors begin degrading and lose their ability to function. This is partially how a tolerance to THC is acquired. These receptors have to regenerate over time. Heavy cannabis use does not allow that to happen. Eventually, some patients report that even using potent THC concentrates yields diminished effects, and this is due the number of active CB1 receptors in the brain being reduced.7
A common strategy for overcoming a tolerance to THC is simply to use more which escalates the degradation of CB1 receptors. Another option is taking a tolerance break (refraining from use)8, but this can be difficult in cases of chronic pain. Alternatively, changing strains is widely reported as a solution to this common problem. Cannabinoids can augment CB1 receptors making them more sensitive or better at generating a signal to the nervous system.9,10 Different cannabinoids such as THCV can also have entirely different effects when binding to the CB1 receptor such as appetite suppression.11 Users of THCV isolates often report feeling a different anxiety free effect, however this is short-lived.12 CBC and CBN are also reported to weakly bind to the CB1 receptor, however they predominantly interact with the CB2 system, and are believed to provide more of an anti-inflammatory effect (See Figure 1 from reference 13).13
To date over 150 cannabinoids have been identified in cannabis plants, however commercial interest is currently focused on measuring 11 of the most common (CBDA, CBD, CBDV, CBGA, CBG, CBC, CBN, THCA, d9-THC, d8-THC, and THCV) excluding synthetic compounds. Many other cannabinoids are present in trace amounts and are not commonly measured due to lack of available standards and how low their percent weight compositions are relative to THCA, THC, CBDA and CBD. Although their presence is not always indicated on a certificate of analysis, minor cannabinoids are always present in full spectrum products. THC and CBD isolate products have fewer pathways to interact with the body which leads to increased tolerance in patients. This makes increasing dosage a poor long term strategy however alternating strains may increase cannabinoid efficacy and require smaller amounts.
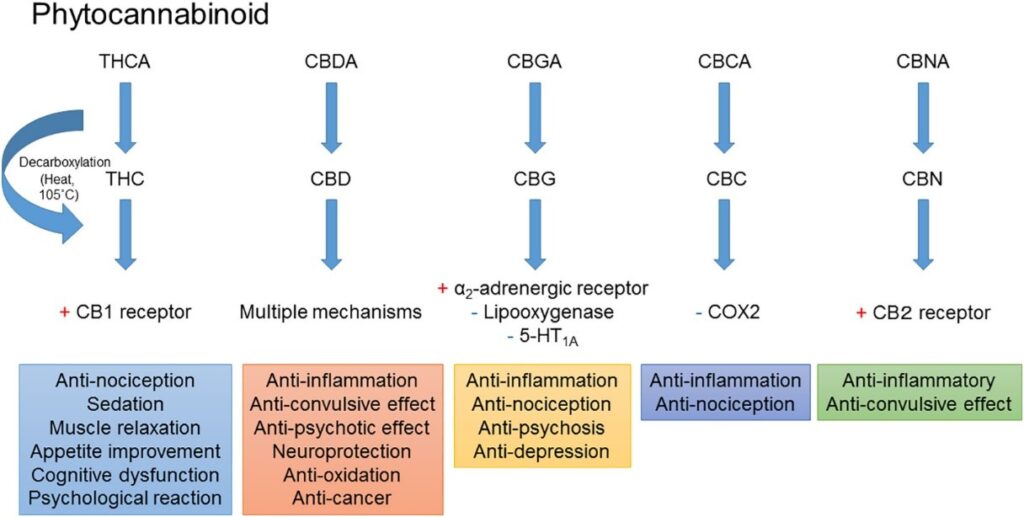
Figure 1: Phytocannabinoid effects and mechanisms from Zaid et al.
References
- Pertwee, R. G. Pharmacology of Cannabinoid CB1 and CB2 Receptors. Pharmacol. Ther. 74(2). 1997, 129-180. https://doi.org/10.1016/S0163-7258(97)82001-3.
- Bayewitch, M., Rhee, M. H., Avidor-Reiss, T., Breuer, A., Mechoulam, R., Vogel, Z. (—)-Δ9-Tetrahydrocannabinol Antagonizes the Peripheral Cannabinoid Receptor-mediated Inhibition of Adenylyl Cyclase. J. Biol. Chem. 1996. 271(17), 9902-9905. https://doi.org/10.1074/jbc.271.17.9902.
- Shao, Z., Yin, J., Chapman, K., Grzemska, M., Clark, L., Wang, J., Rosenbaum, D. M., High-Resolution Crystal Structure of the Human CB1 Cannabinoid Receptor. Nature. 2016. 540, 602–606. https://doi.org/10.1038/nature20613
- Zurier, R. B., Prospects for Cannabinoids as Anti-Inflammatory Agents. J. Cell Biol. 2003, 88(3). 462-466. 10.1002/jcb.10291
- Aly, E., Khajah, M. A., Masocha, W. β-Caryophyllene, a CB2-Receptor-Selective Phytocannabinoid, Suppresses Mechanical Allodynia in a Mouse Model of Antiretroviral-Induced Neuropathic Pain. Molecules. 2020. 25(1), 106. 10.3390/molecules25010106
- Ceccarelli, I., Fiorenzani, P., Pessina, F., Pinassi, J., Aglianò, M., Miragliotta, V., Aloisi, A. M. The CB2 Agonist β-Caryophyllene in Male and Female Rats Exposed to a Model of Persistent Inflammatory Pain. Front. Neurosci. 2020. 14, 850. 10.3389/fnins.2020.00850
- Daigle, T. L., Kearn, C.S., Mackie, K. Rapid CB1 Cannabinoid Receptor Desensitization Defines the Time Course of ERK1/2 MAP Kinase Signaling. Neuropharmacology. 2008. 54(1), 36-44. 10.1016/j.neuropharm.2007.06.005
- Hirvonen, J., Goodwin, R. S., Li, C-T., Terry, G. E., Zoghbi, S. S., Morse, C., Pike, V. W., Volkow, N. D., Huestis, M A., Innis, R. B. Reversible and Regionally Selective Downregulation of Brain Cannabinoid CB1 Receptors in Chronic Daily Cannabis Smokers. Mol. Psychiatry. 2012. 17, 642–649. https://doi.org/10.1038/mp.2011.82
- Thomas, A., Baillie, G. L., Phillips, A. M., Razdan, R. K., Ross, R.A., Pertwee, R.G. Cannabidiol Displays Unexpectedly High Potency as an Antagonist of CB1 and CB2 Receptor Agonists in Vitro. Br. J. Pharmacol. 2007. 150(5), 613-623. doi:10.1038/sj.bjp.0707133
- Laprairie, R. B., Bagher A. M., Kelly M. E., Denovan-Wright, E. M. Cannabidiol Is a Negative Allosteric Modulator of the Cannabinoid CB1 Receptor. Br J Pharmacol. 2015. 172(20), 4790-805. 10.1111/bph.13250.
- Abioye, A., Ayodele, O., Marinkovic, A., Patidar, R., Akinwekomi, A., Sanyaolu, A. Δ9-Tetrahydrocannabivarin (THCV): A Commentary on Potential Therapeutic Benefit for the Management of Obesity and Diabetes. J. Cannabis Res. 2020. 2, 6. 10.1186/s42238-020-0016-7
- Englund, A., Atakan, Z., Kralj, A., Tunstall, N., Murray, R., Morrison, P. The Effect of Five Day Dosing with THCV on THC-Induced Cognitive, Psychological and Physiological Effects in Healthy Male Human Volunteers: A Placebo-Controlled, Double-Blind, Crossover Pilot Trial. J. Psychopharmacol. 2016. 30(2), 140-151. doi:10.1177/0269881115615104
- Zaid, H. M., Maayah, Takahara, S., Ferdaoussi, M., Dyck, J. R. B., The Molecular Mechanisms that Underpin the Biological Benefits of Full-Spectrum Cannabis Extract in the Treatment of Neuropathic Pain and Inflammation. Biochim. Biophys. Acta – Mol. Basis Dis. 2020.1866(7), 165771. https://doi.org/10.1016/j.bbadis.2020.165771